Article content
The European Medicines Agency warns everyone about the impending danger posed by these medicines. This institution has already ordered the cessation of production and the suspension of the use of the medicines in all hospitals and pharmacies. This drug is already responsible for 12 serious brain illnesses and 3 of these cases were fatal.
Dangerous drug
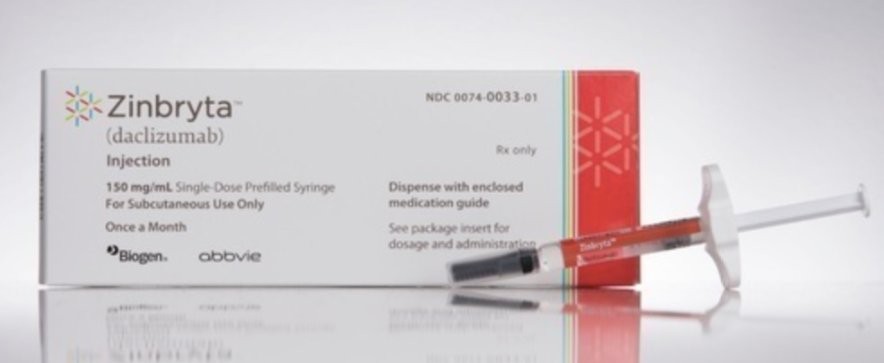
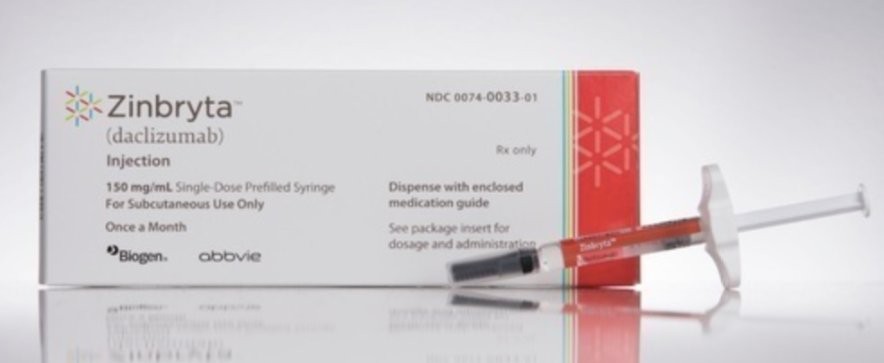
The European medicines regulator recommended the suspension of the distribution of that drug with immediate effect. It concerns the drug Zinbrya.
This drug was originally intended for the treatment of multiple sclerosis. The drug has already been withdrawn from sale.
This serious measure was issued on the basis of 12 very serious cases of brain disease.
The order also applies to all pharmacies and hospitals so that this drug is no longer sold or administered to patients.
All physicians are obliged to inform their patients who take Zinbrya of this fact. The best option is to stop taking this drug immediately and, after consulting your doctor, replace it with another alternative.
Patients who will have to stop taking this drug should be closely monitored by their doctor for at least 6 months.
Affected organs
Most recorded cases manifested within 8 months of starting treatment with this drug.
Since 2017 there have been cases known in which this drug was associated with severe immune-mediated liver disease. This disease can also manifest up to 6 months after stopping treatment with this drug.
Other conditions associated with this drug include blood disorders, thyroid inflammation, or kidney inflammation.