Article content
The State Institute for Drug Control (SÚKL) informed the public about the withdrawal of the product at the level of distribution companies, pharmacies and patients. It acted for the purpose of protecting the health and safety of the patient.
Many of us are accustomed to using various nasal sprays that ease breathing and reduce mucosal swelling.
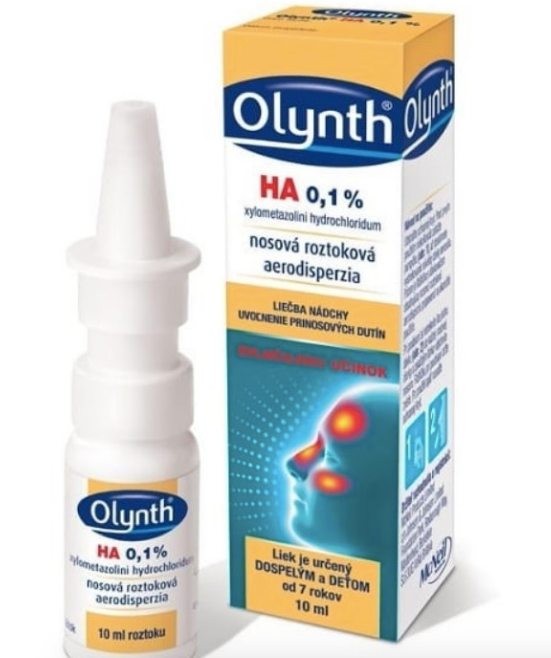
The State Institute for Drug Control announced on its website the withdrawal of a nasal spray.
“For reasons of protecting the health and safety of the patient, all batches of the product manufactured since October 1, 2018 are being withdrawn. Those particularly at risk are patients with impaired immune system function and sensitive individuals. The State Institute for Drug Control urges patients to stop using the preparation Olynth HA 0.1%,” the institute states.
The State Institute for Drug Control discovered irregularities with the product during inspection, which reduces swelling of the nasal mucosa in acute rhinitis, mucus production and in allergic rhinitis.
The reason is said to be a dangerous risk of microbial contamination.
Specifically, it concerns batches F144450A and F144460A, and patients may return the given medicine to the pharmacy.